What Is NAD+
NAD+, short for nicotinamide adenine dinucleotide, is the oxidized form of NADH. It’s main biological function is to carry electrons from one biochemical reaction to another, acting to shuttle energy within a cell and, in certain conditions, to extracellular locations as well. NAD+ also plays roles in enzyme activation/deactivation, posttranslational modification of proteins, and cell-to-cell communication. As an extracellular signaling molecule, NAD+ has been found to be released from neurons in blood vessels, the bladder, the large intestine, and from certain neurons in the brain.
NAD+ Structure
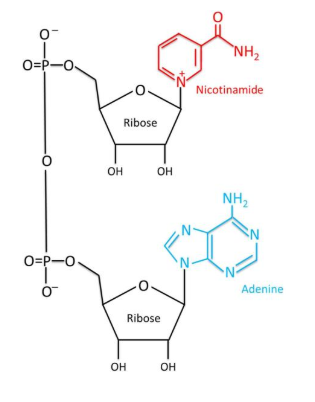
Sequence: N/A
Molecular Formula: C21H27N7O14P2
Molecular Weight: 663.43 g/mol
PubChem CID: 925
CAS Number: 53-84-9
Synonyms: nicotinamide adenine dinucleotide, beta-NAD, NAD, Endopride
NAD+ Effects
- NAD+ is best thought of as a support molecule that is essential to cellular metabolism as well as extracellular communication. Research shows that NAD+ plays important roles in energy conversion, DNA repair, immune defense, and circadian cycles. Levels of the cofactor, however, are sensitive to disease state as well as age. NAD+ as the following effects that decline as a result of natural age-related decreases in the levels of the cofactor.
- NAD+ activates sirtuins and other enzymes, liked Poly-ADP-ribose polymerases, involved in DNA repair and inflammatory processes. Sirtuins are the same enzymes linked to the life-extending benefits of calorie restriction.
- NAD+ controls the production of the protein PGC-1-alpha, which protects neurons and other cells in the central nervous system from oxidative stress. Research in mice shows that this particular effect may be linked to improved memory, particularly with aging.
- In mouse models, NAD+ helps to protect blood vessels against age-related hardening and the deposition of atherosclerotic plaques. In some studies, the cofactor even helps to reverse age-related dysfunction of the aorta.
- Mice given NAD+ show increased rates of metabolism and improved lean body mass.
- Increased NAD+ levels can increase muscle strength and endurance in older mice.
- NAD+ has been linked to extracellular signaling, particularly for smooth muscle. It may be of benefit in GI function. This effect is likely responsible for NAD+ benefits on blood pressure[1], [2].
NAD+ Additions and Synergies
- Because NAD+ is a naturally occurring molecule, it is easy to combine with other supplements to obtain synergistic effects with few to no side effects. This is particularly true when NAD+ is combined with other natural supplements. Research in mice bears this out in several specific cases.
- Combining NAD+ and high-dose biotin may help to combat pain and reduce levels of pain.
- CoQ10, another cofactor in energy metabolism, may work synergistically with NAD+ to improve neurological function and protect the central nervous system against oxidative stress[3].
- Reservatrol and NAD+ make work together to reduce oxidative damage, lower inflammation, and help to lower levels of LDL (a.k.a. bad) cholesterol. They may also work together to protect against diabetes and neurodegenerative disease[4].
- Vitamins B1, B2, and B6 help to boost NAD+ salvage. When combined with NAD+ supplementation, they may help to improve overall NAD+ levels.
- Combining NAD+ with mitochondrial and energy supplements, such as creatine and alpha-lipoic acid, may boost antioxidant and anti-aging effects.
NAD+ Research
Anti-Aging Research and NAD+
One of the primary results of the standard aging process is a decline in both the quality and activity of mitochondria. Mitochondria are the body’s power plants, producing the energy for everything from neuron firing to digestion and muscle function. A decline in mitochondrial functioning has been associated with normal aging, but is also a factor in a number of age-related disease processes. Research shows that mitochondrial aging contributes to cellular senescence, inflammation, and even changes in stem cell activity that reduce rates of healing and make it harder for the body to recover from injury in old age[5].
According to Nuo Sun of the Heart, Lung, and Blood Institute of the National Institutes of Health, mitochondria cannot simply be viewed as bioenergetics factories, but “rather as platforms for intracellular signaling, regulators of innate immunity and modulators of stem cell activity.” He goes on to explain that “mitochondria can be linked to a wide range of processes associated with aging including senescence, inflammation, as well as the more generalized age-dependent decline in tissue and organ function.” In other words, mitochondria are the lynch pin of cellular aging and understanding how to protect their function is a necessary first step in understanding how to slow, stop, or even reverse the aging process.
The Role of NAD+ In Muscle Function
Another link between aging and NAD+ can be seen in skeletal muscle tissue. In mouse models, age-related muscle decline occurs in two steps. In the first step, oxidative phosphorylation (the process mitochondria use to produce energy) declines because of reduced expression of mitochondrial genes (mitochondria contain their own DNA). In the second step, genes regulating oxidative phosphorylation begin to malfunction in both the mitochondria and nucleus. Phase 1 is reversible. If NAD+ is administered, mice in these studies show improved mitochondrial function and do not progress to step 2. If, however, the mice are allowed to progress to stage 2 without intervention, then NAD+ cannot rescue them[9]. This evidence suggests that intervention in mitochondrial aging is possible using NAD+, but that waiting too long results in refractory dysfunction. It is the best argument yet that early supplementation with NAD+ is critical to fighting off aging in the long term.
Research shows that exercise training actually has the same effects on aging mitochondria as NAD+ supplementation does. It appears that, in both cases, intervention helps to prevent changes in peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC-1-alpha) signaling that lead to mitochondrial dysfunction[10].
Research in mouse models of skeletal muscle aging suggests that exercise training helps to maintain muscle oxidative capacity over a lifetime. At least part of the reason that this works is that exercise increases PGC-1-alpha levels, which in turn helps to protect mitchondrial DNA, oxidative proteins, and angiogenic (blood vessel stimulating) proteins[11].
NAD+ in Neurodegenerative Disease
Much of what has been learned about NAD+ and the aging process is actually applicable to a number of disease conditions. In particular, changes in NAD+ appear to have far-reaching effects in the central nervous system and have been linked to a number of neurodegenerative diseases such as Alzheimer’s and Huntington’s diseases. A review article published in 2019 explained the current state of the knowledge as it relates to NAD+ and the central nervous system. In short, NAD+ is neuroprotective in a number of mouse models of human diseases such as Huntington’s disease. It appears that the cofactor is important in improving mitochondrial function, which in turn decreases the production of reactive oxygen species (ROS). ROS are known to cause damage in a number of inflammatory and disease conditions. They also accelerate the aging process. There is interest in a possible synergistic effect that could be gained through NAD+ supplementation in combination with a class of medications known as PARP inhibitors. PARP proteins are involved in DNA repair and programmed cell death. Though activated PARP is important to DNA repair, too much PARP activity can actually deplete cellular energy stores and induce programmed cell death[12].
Research in mouse models of Parkinson’s disease shows that NAD+ supplementation helps to protect against motor deficits and the death of dopaminergic neurons in the substantia nigra. This suggests that NAD+ may not only help ameliorate the symptoms of Parkinson’s disease, but may actually slow or even prevent the development of the disease in the first place[13].
Interesting research into a metabolic process known as the kynurenine pathway (KP) has shown that NAD+ supplementation may help to ward off disease by preventing the breakdown of neurotransmitters and by reducing the need to shunt protein precursors to the production of NAD+. Tryptophan is an essential amino acid and is a building block of a number of neurotransmitters and proteins. This amino acid is broken down, however, via the KP to produce NAD+. Thus, the production of NAD+ directly cannibalizes essential neurotransmitters. Research has linked imbalances in KP activity to Parkinson’s, Alzheimer’s, and Huntington’s diseases as well was psychiatric disorders like schizophrenia and bipolar disorder[14]. There is ongoing research to determine if NAD+ supplementation can prevent imbalances in KP and thus ameliorate or prevent the neurodegenerative conditions mentioned.
The Role of NAD+ in Reducing Inflammation
NAD+ levels are regulated by a number of factors, one of which is NAMPT. This particular enzyme is known to be associated with inflammation and is often overexpressed by certain types of cancer. Researchers are, in fact, targeting NAMPT as a potential anti-cancer treatment. The regulator has also been linked to the development of obesity, type 2 diabetes, and nonalcoholic fatty liver disease. It is a potent activator of inflammation and its levels increase dramatically as NAD+ levels decrease. It is thought that supplementation with NAD+ can help to reduce NAMPT activation and thus modulate inflammation[15].
There is good evidence to suggest that the NAD+/NAMPT dichotomy is a primary driver of the insulin resistance that has been linked to obesity and so often leads to type 2 diabetes as well as heart disease. It appears that obesity leads to inflammation and that leads to an overall reduction in NAD+ levels, which in turn increases free fatty acid levels in the blood as a result of adiponectin down-regulation. This then causes the liver to produce more glucose even as it interferes with the insulin-mediated uptake of glucose by skeletal muscle. The result is insulin resistance, which the pancreas attempts to overcome by producing more insulin. The net result, over time, is high glucose levels and diabetes[16].
NAD+ Supplementation and the Future of Aging Research
There is good evidence from animal models to suggest that NAD+ supplementation can offset some of the effects of mitochondrial aging. Much of this evidence, however, comes from animal models. There has been a strong push to test NAD+ in clinical trials of neurodegenerative disease and chronic type 2 diabetes. In both cases, the simple cofactor holds a great deal of promise for, at the very least, slowing the progression of these devastating diseases. There is even hope that NAD+ can, by itself or in combination with other therapies, reverse certain disease processes or even regulate the aging process itself.
 USD
USD EUR
EUR GBP
GBP CAD
CAD





