AICAR, short for 5-aminoimidazole-4-carboxamide ribonucleoside, is short peptide that plays a role in energy homeostasis and a number of metabolic pathways. AICAR plays a role in the regulation of insulin receptors and how muscle cells function with regards to insulin. AICAR is under active investigation for its cancer-fighting properties and for its ability to protect heart/cardiovascular tissue. AICAR is an AMP kinase (AMPK) activator.
AICAR is the activated form of naturally occurring acadesine, which is currently used in the treatment of acute lymphblastic leukemia. Research shows that acadesine, like AICAR, has anti-cancer properties. It has also been found to play a role in inhibiting platelet function and thus in the prevention of the early stages of blood clotting.
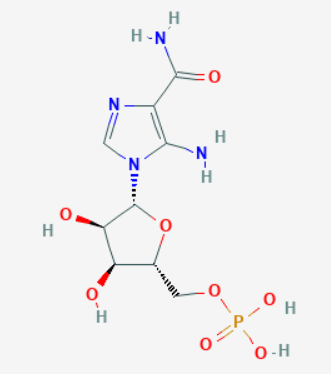
Source: PubChem
Sequence: 5-aminoimidazole-4-carboxamide ribonucleoside
Molecular Formula: C9H15N4O8P
Molecular Weight: 338.213 g/mol
PubChem CID: 65110
CAS Number: 3031-94-5
Synonyms: AICA ribonucletotide, Z-nucleotide
Research in mice shows that AICAR, even at low doses, reduces inflammation in adipose tissue. Inflammation in fat is associated with increased insulin resistance and reducing inflammation leads to improved glucose homeostasis and increased insulin sensitivity even without any changes in body weight. I appears that AICAR has several pathways though which it affects inflammation in adipose tissue, with at least one of those pathways involving SIRT1 and macrophages[1].
The impact of AICAR on adipose inflammation is not unexpected. AMPK has been found to attenuate inflammatory responses in metabolic disorders in both healthy and diabetic mice. In research in mice, AMPK activation, as is caused by AICAR, was found to improve insulin sensitivity, energy homeostasis, lipid metabolism, and inflammatory markers[].
Exercise increases the number of GLUT-4 insulin receptors that are present on the surface of muscle cells. It is one of the most effective means of boosting glucose uptake by muscle cells and effectively reduces both glucose levels and insulin resistance. It turns out that AICAR mimics the effects of exercise very precisely and that repeated administration of AICAR has effects similar to long-term exercise[3].
AMPK plays a complex role in the growth and metastasis of cancer, both slowing and accelerating the growth of tumors under varying circumstances. Overall, research indicates that prolonged activation of the enzyme eventually leads to cancer cell death by slowing cancer cell metabolism and making cancer cells more susceptible to environmental insults. This has been demonstrated both in cell culture and in rats[4, p. 5], [5]. Scientists are investigating the ability of AICAR to work in tandem with other chemotherapeutic agents to boost effectiveness. The thought is that AICAR might:
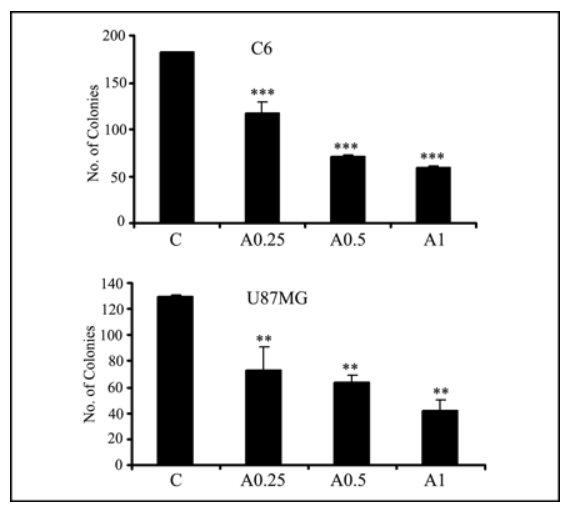
AICAR inhibits clonal growth of glioma (C6) and prostate cells.
Source: Journal of Biological Chemistry
Research in thyroid cancer cells indicates that AICAR may also operate by causing apoptosis (programmed cell death). It appears that this activity is mediated through the induction of p21 accumulation and the eventual activation of caspase 3. The overall effect is inhibition of cancer cell proliferation and survival[7].
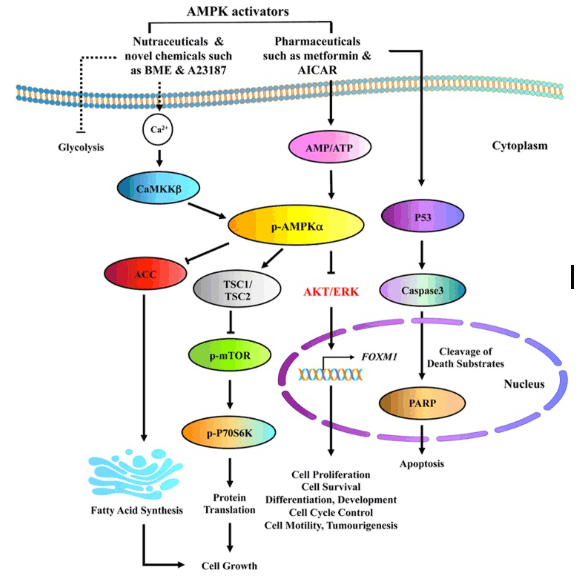
AMPK activators, like AICAR, influence a number of pathways that can impact cancer growth.
Source: PubMed
AMPK activators have been shown to play an important role in inflammation at the cellular level. Research into metformin, a common and long-used diabetes medication, indicates that at least part of the reason the drug is effective is that it reduces inflammation and boosts the function of the pancreas. AICAR has a similar effects, playing a protective role in inflammatory conditions like acute lung injry, asthma, colitis, atherosclerosis, and hepatitis[8].
There is ongoing research into the use of AICAR to mediate the effects of auto-immune diseases and other inflammatory conditions. For instance, studies in mice indicate that ACIAR may be effective in reducing inflammation in colitis. It appears that AICAR acts as a central inhibitor of immune responses in this setting by reducing NF-kappaB activation in macrophages as well as TH1- and TH17-type cytokines[9].