Adipotide was developed and placed into phase I clinical trials in 2011 to investigate its ability to kill fat cells. Tests in rhesus monkeys revealed that adipotide causes targeted apoptosis in the blood vessels of white adipose tissue (fat). Without a blood supply, the fat cells simply died. The net result was rapid weight loss, rapid decrease in body mass index (BMI), and improved insulin resistance characteristics. Interestingly, treatment with adipotide and subsequent fat loss not only improved weight, but actually contributed to changes in eating behavior. Monkeys who lost weight with adipotide also showed a decrease in food consumption[1].
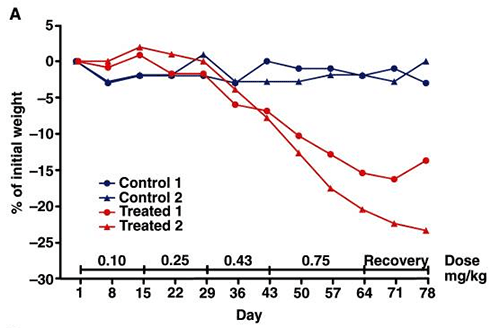
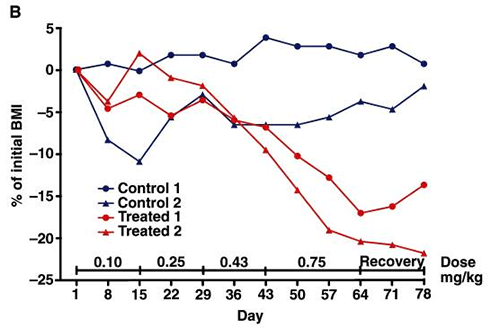
A. Percent weight loss in control groups (blue) versus those treated with adipotide (two differing doses, shown in red)
B. Percent reduction in BMI (control versus treatment)
Source: PubMed
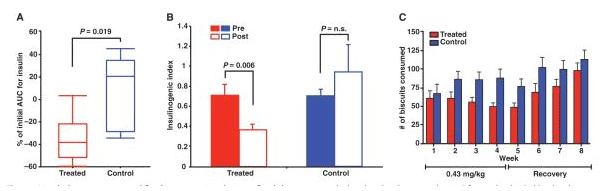
A. Shows the change in insulin requirements (area under the curve) for treated (red) and control (blue) groups. The AUC was calculated from an IVGTT test.
B. Shows the insulinogenic index for before and after in the treatment (red) and control (blue) groups. Treated groups show a dramatic decrease in insulin secretion.
C. Change in biscuit consumption in treated (red) and control (blue) groups.
Source: PubMed
The targeting of adipotide to the blood vessels serving fat cells may be mediated by a protein receptor called prohibitin. Prohibitin is a membrane protein that may only be found in blood vessels serving white fat and in cancer cells. It has been demonstrated that adipotide associates with this protein[2]. If it turns out that prohibitin is found only in fat vasculature and cancer tissue, then adipotide testing will be responsible for identifying a fat-specific target that can be used not just for therapeutic purposes, but for diagnostic purposes as well.
Prohibitin, the molecule that adipotide likely targets in fat cells, has been associated with certain types of cancer[3]. Cancer cells are known to require substantial blood supplies in order to grow and metastasize. The ability to target prohibitin in cancer cells may provide for advanced therapies that target cancer without harming surrounding tissues.
Glucose tolerance is a term that refers to higher than normal levels of blood sugar. The condition is generally diagnosed with a blood test and confirmed by testing fasting glucose levels or by administering a glucose tolerance test in which a set amount of sugar is consumed and then blood levels of sugar are measured. Glucose tolerance is a proxy measure for diabetes, with increased glucose tolerance indicating a trend toward diabetes.
Treating elevated glucose levels can be done with diet and exercise, but these methods require dedication and motivation. They also take considerable time to have an effect. In general, most people with impaired glucose tolerance go on to develop overt type 2 diabetes and require drugs like metformin and, in some cases, insulin. Research into adipotide has revealed that the peptide produces rapid and weight-independent improvement in glucose tolerance[4]. The latter part is critical, because it indicates that a reduction in white fat by adipotide is effective in reducing glucose tolerance regardless of the impact on weight. In other words, it is the fat loss that is important, not the weight loss. These findings not only open the pathway for developing new treatments for pre-diabetes and diabetes, they help to clarify and explain the mechanisms that lead to diabetes in the first place.
There is some argument as to whether adipotide directly causes fat loss or simply decreases food intake which indirectly leads to fat loss[4]. It is likely that adipotide directly causes fat loss. This hypothesis is supported by the fact that the peptide causes changes in fat cell density without causing weight loss and affects glucose tolerance without causing weight loss.